What is Medical Affairs?
Medical Affairs department in pharmaceutical company has been around for over 50 years.
Medical Affairs is a function that has existed for more than 50 years in Western pharmaceutical industries. Our consulting clients sometimes say to us, “Medical Affairs is a new organization, isn’t it?” Actually, this is not true at all. Medical Affairs already existed decades ago in major foreign pharmaceutical companies operating in Japan; however, human resources were rather limited in those days and Medical Affairs also played limited roles, such as preparing a protocol for clinical research to be conducted in Japan jointly with their global headquarters, negotiating with a physician who would assume the director of the clinical research, etc.
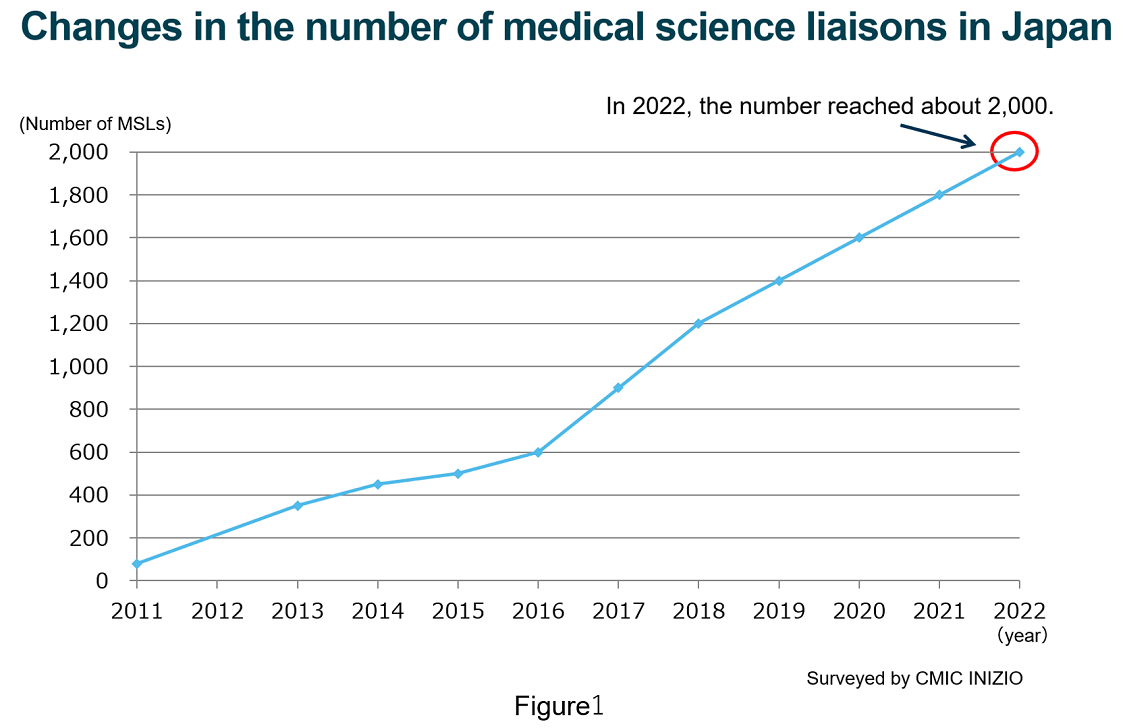
The number of MSLs in Japan has reached 2,000.
It is just recently that Medical Affairs has attracted attention in Japan. Do you know why?
In Japan, pharmaceutical companies has increased human resources in Medical Affairs. They recently assigned dozens of people, sometimes over a hundred people to work in Medical Affairs, especially to play a role called a medical science liaison (MSL). Our survey has shown that about 2,000 people currently act as MSLs across Japan(Figure 1).
In Japan, pharmaceutical companies has increased human resources in Medical Affairs. They recently assigned dozens of people, sometimes over a hundred people to work in Medical Affairs, especially to play a role called a medical science liaison (MSL). Our survey has shown that about 2,000 people currently act as MSLs across Japan(Figure 1).
Why have pharmaceutical companies in Japan rapidly increased the number of personnel in their Medical Affairs departments?
Do you know why they increased the number of MSLs?
We believe that they had two main reasons. First, while global pharmaceutical companies are committed to implement social contribution activities, Japanese companies probably found it necessary to follow their strategy. Second, as you may remember, a pharmaceutical company was found to have falsified clinical datai) and this may have influenced the change in Medical Affairs in Japan. A statistician of the company was involved in the falsification of clinical data, which was obtained from an investigator-initiated clinical trial of the company’s drug for the treatment of hypertension. A series of published articles were withdrawn because they were affected by the falsification. This was attributed to a conflict of interest between the company and relevant healthcare professionals and developed into a serious problem that jolted the whole industry. After this shock, other companies began to feel the necessity of establishing an independent department involved in the conduct of clinical research and associated prior communication with physicians, separately from other departments involved in marketing and other commercial activities. This led to the recent rapid expansion of Medical Affairs resources, especially those serving as MSLs. As employees not directly involved in the sales of their company’s products, MSLs are expected to communicate with physicians about specific disease areas from a sophisticated scientific perspective. In view of these purposes, the activities of MSLs are considered to require medical or pharmaceutical experiences and the assignment of such resources is recommended across the industry.ii)
Under these circumstances, Japanese pharmaceutical companies also started to organize departments called Medical Affairs or Ikuyaku (a Japanese word meaning “post-marketing drug development”), although these organizations have been considered to be unique to foreign companies.
We believe that they had two main reasons. First, while global pharmaceutical companies are committed to implement social contribution activities, Japanese companies probably found it necessary to follow their strategy. Second, as you may remember, a pharmaceutical company was found to have falsified clinical datai) and this may have influenced the change in Medical Affairs in Japan. A statistician of the company was involved in the falsification of clinical data, which was obtained from an investigator-initiated clinical trial of the company’s drug for the treatment of hypertension. A series of published articles were withdrawn because they were affected by the falsification. This was attributed to a conflict of interest between the company and relevant healthcare professionals and developed into a serious problem that jolted the whole industry. After this shock, other companies began to feel the necessity of establishing an independent department involved in the conduct of clinical research and associated prior communication with physicians, separately from other departments involved in marketing and other commercial activities. This led to the recent rapid expansion of Medical Affairs resources, especially those serving as MSLs. As employees not directly involved in the sales of their company’s products, MSLs are expected to communicate with physicians about specific disease areas from a sophisticated scientific perspective. In view of these purposes, the activities of MSLs are considered to require medical or pharmaceutical experiences and the assignment of such resources is recommended across the industry.ii)
Under these circumstances, Japanese pharmaceutical companies also started to organize departments called Medical Affairs or Ikuyaku (a Japanese word meaning “post-marketing drug development”), although these organizations have been considered to be unique to foreign companies.
What is the purpose of the Medical Affairs department?
Next, let’s think about what are the purposes of Medical Affairs organizations.
People working in these organizations are always considered to be not directly committed to increasing the sales of their company’s products. Do you think this is true?
We can summarize the purposes and activities of Medical Affairs as follows:
People working in these organizations are always considered to be not directly committed to increasing the sales of their company’s products. Do you think this is true?
We can summarize the purposes and activities of Medical Affairs as follows:
(1) Identify unmet medical needs (UMNs) in relevant disease areas;
(2) Determine appropriate measures to address these needs; and
(3) Actually implement these measures.
What is an unmet medical need?
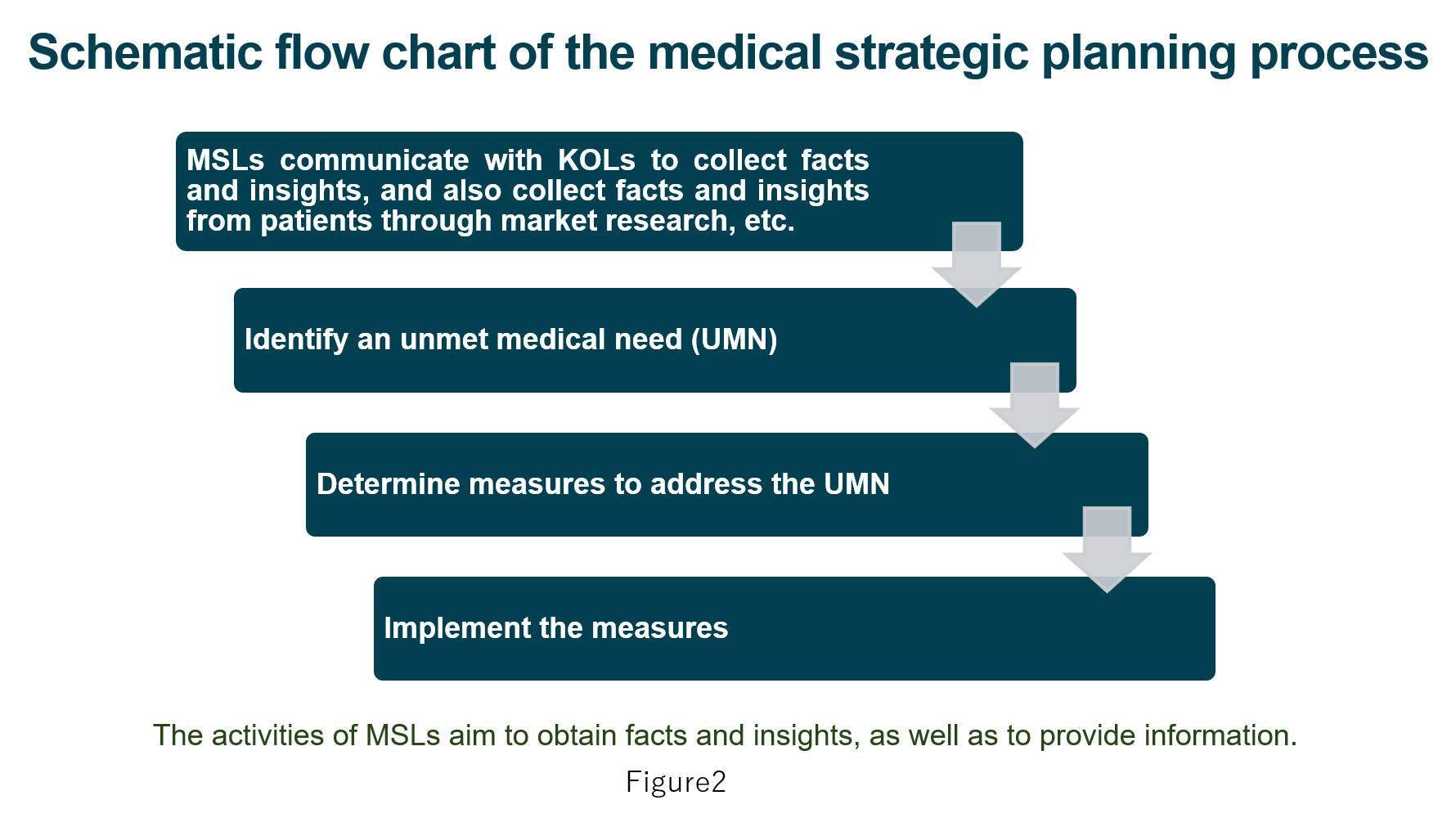
Identifying UMNs is one of the important roles of MSLs. They communicate with physicians who are regarded as key opinion leaders (KOLs)iii) in relevant disease areas to obtain information on current issues they are facing there. This information is called a fact or insight (you can see more details in the column). MSLs review the information to identify unmet medical needs. At this stage, they must note that it is critical to collect information from individual patients, as well as physicians, because they are all customers of their company. I will explain how MSLs collect information separately in the column. MSLs now review the collected information and when they identify an UMN, they seek appropriate measures to address it(Figure 2).
The Medical Strategic Plan is the cornerstone of the Medical Affairs department’s activities.
These steps of their activities are often shown in a flow chart, which is called a medical strategic plan.
In most cases, the conduct of clinical research is needed to address an UMN and Medical Affairs plays a major role in planning this research. Based on the information collected by MSLs, in-house Medical Affairs staff members develop a strategy for clinical research and implement it. This is the general process for Medical Affairs. Meanwhile, MSLs may also serve as in-house staff members in some companies.
In most cases, the conduct of clinical research is needed to address an UMN and Medical Affairs plays a major role in planning this research. Based on the information collected by MSLs, in-house Medical Affairs staff members develop a strategy for clinical research and implement it. This is the general process for Medical Affairs. Meanwhile, MSLs may also serve as in-house staff members in some companies.
The objective of the Medical Affairs department is to obtain medium- and long-term profits.
As mentioned above, Medical Affairs is considered to be not directly committed to increasing the sales. This is actually true, but more specifically, they are not involved in a short-term sales strategy, but involved in a middle- or long-term sales strategy to increase the profit of their company. This is a more correct description of this organization.
I will also post more details of this point in the column. Please check it out later.
I will also post more details of this point in the column. Please check it out later.
CMIC INIZIO Co., Ltd.
Hiroyuki Tanaka,
Vice president and the head of Medical Affairs Company
i)The Asahi Shimbun. Top news events in 2016 (online).
(http://www.asahi.com/articles/DA3S12676631.html, latest access on December 20, 2020)
ii)The Japanese Association of Pharmaceutical Medicine. Medical Science Liaison-Board Certification program.
(https://japhmed.jp/msl/msl.html, latest access on December 20, 2020)
iii)KOLs are also called scientific thought leaders (STLs), key external experts (KEEs),etc.